Background: ChromaTwist Dyes for Flow Cytometry: The chemical modularity of ChromaTwist fluorescent dye technology has allowed 50+ UV excitable fluorescent dyes to be created that emit across the visible spectrum (395-635nm, and potential for reaching into IR emission), without the need for tandem dye based approaches, and their drawbacks for end-users. Previously we demonstrated that the ChromaTwist blue dye-antibody (CD4 & CD8) conjugates have utility in both multicolour and spectral flow cytometry (BD Bioscience Fortessa X-20, Cytek Aurora (355nm); Sony Biotechnology ID7000 (320nm, 355nm), ThermoFisher BigFoot (355nm)).
Potential of the ChromaTwist UV Excitable Dye Technology in Flow Cytometry:
Multicolour Flow Cytometry: The chemical tunability of the ChromaTwist dyes means that we are able to match the fluorescent emission outputs to the 6 visible flow cytometry filters and ultimately the 2 IR filters, and bring to the multicolour market a portfolio of UV excitable molecular based dyes, without the end-user drawbacks associated with tandem polymer dyes.
UV Spectral Flow Cytometry: The chemical tunability of the ChromaTwist dyes means that we will be able to go far beyond the multicolour 8 dye range and be able to present a portfolio of 50+ UV excitable dyes (320nm and/or 355nm), each with a unique spectral fingerprint. Ultimately this means ChromaTwist could offer 50+ ChromaTwist-dye antibody conjugates which are all excited by 320 and/or 355nm UV laser.
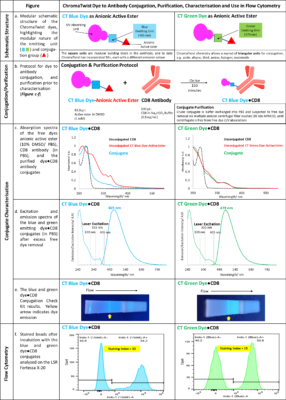
Dye Structure, Figure a: Schematic structures of the dyes, as the anionic active esters, consist of the UV absorbing unit, fused to the aromatic blue or green emitting unit, which in turn is linked via a short linker to the anionic active ester. The emitting (square unit) can be substituted easily in the synthetic procedure which has allowed, to date, 50+ dyes to be synthesised, each with a different emission colour. In addition, the active ester (traingle unit) can easily be substituted for azide, thiol, alkyne, halogen, amine, alcohol, tosyl and other chemistries.
Dye to Antibody Conjugation and Purification Protocol, Figure b (experimental at end of document): The active ester (in DMSO) is added in excess to the CD8 antibody in sodium bicarbonate buffer and incubated on ice for 180 minutes. DMSO removal and buffer exchange is achieved via 7-8 amicon centrifugal filter washes.
Comparison of Dye●CD8 Conjugate Absorption Spectra to Free Dye and Free Antibody, Figure c: The two purified dye-antibody conjugates absorption spectra are compared to the free antibody and free dye as active ester in Figure c, revealing the conjugate has an absorption profile containing elements of the free dye and free antibody, and a significant shift in the antibody lmax, giving good evidence of conjugation.
Purified Dye●CD8 Conjugate Absorption and Emission Spectra, Figure d: The absorption spectra of both dyes show that they are excited by the conventional 355 nm laser used in flow cytometry, but also the 320 nm laser recently developed by Sony Biotechnology in their ID7000 instrument. Of note is that the 405 nm absorption has been reduced significantly in the blue dye. This reduction is due to the attachment of the electron donating oxygen to the blue emitting unit. The green dye reduction at 405 nm is less, and it is anticipated that this can be corrected by moving the oxygen atom in the green emitting unit. Furthermore, the ChromaTwist absorption profile extends below 300 nm, which holds out the possibility of a differentiated dye set to commercial tandem dyes, as even shorter wavelength lasers are integrated into flow cytometers in the future.
Dye●CD8 Conjugate Detection using a Conjugate Check Kit, Figure e: Solutions of the dye●CD8 conjugates were run on a Conjugation Check Kit (Abcam ab236554). Successful conjugation is evident from the fluorescence observed at the “Test line” upon irradiation with 302 nm light, indicating that the CD8 antibody was indeed labelled with the ChromaTwist dyes.
Dye●CD8 Conjugate Performance on a BD Fortessa X20 Flow Cytometer with Compensation Beads, Figure f: The conjugates in PBS buffer were incubated with compensation beads (25 µL, UltraComp eBeads™), for 30 minutes (22 °C, in the dark), were washed with cell staining buffer(2 x 1400 µL), resuspended in cell staining buffer (500 µL) and transferred to FACS tube for analysis on a LSR Fortessa X-20. Beads were gated to exclude debris and doublets and fluorescence is observed in the indo-1 (Violet) and indo-1 (Blue) fluorescence channels for the blue and green CT dye conjugates respectively, which afforded staining indices of 33 (blue conjugate) and 15 (green conjugate).
ChromaTwist is currently working on a development program to push these staining indices up by factors of 2, 3, 4, 6, 8, and 9 and potentially beyond, via a scalable amplification technology.
Experimental: Active ester dye (63.8 µL, DMSO, 1 mM) is added to 50 µg CD8 RPA-T8 (400 µL sodium bicarbonate buffer, 100 mM, pH 8.3 – 8.5), vortexed to mix and incubated for 150 minutes on ice. Buffer exchange into PBS is achieved via 7-8 amicon centrifugal filter washes (30 kDa MWCO). The conjugate (2.5 – 10 µL) is added to compensation beads (25 µL, UltraComp eBeads™ Compensation Beads, Invitrogen cat no. 01-2222-41) and vortexed to mix (5 s). Beads are incubated for 30 minutes (22 °C, in the dark) and washed twice with the addition of cell staining buffer (1400 µL, Biolegend cat no. 420201), centrifugation (600 g, 5 min) and discarding the supernatant. Beads are resuspended with the addition of cell staining buffer (500 µL), vortexed (5 s) and transferred to FACS tube for analysis on a LSR Fortessa X-20 flow cytometer. Beads are gated to exclude debris and doublets and fluorescence is observed in the indo-1 (Violet) and indo-1 (Blue) fluorescence channels for the blue and green CT dye conjugates, respectively.
