Executive Summary
ChromaTwist dyes (CT-Dye) all have a similar excitation spectra from ~260 nm to ~425nm, but can be chemically tuned to emit with emission maxima ranging from 395 nm to over 600 nm with large extinction coefficients (generally >100 000 M-1 cm-1) and quantum yields of ~50%, with ~80 different CT-Dye examples. Therefore, the CT-Dyes can be excited with the three lowest wavelength excitation lasers used in commercial spectral flow cytometers (320nm, 355nm and 405 nm), whilst at the same providing tuneable emission over the full visible spectrum, which can be detected by multiple APDs/PMTs.
This combination of photophysical properties means the CT-Dyes, with their excitation profiles, spanning 3 lasers are potentially ideal dyes for full spectral flow cytometry, as they have the possibility of giving high spectral complexity, and hence differentiation from dyes with similar spectral emission outputs, which in turn affords the potential to extend multiplexing significantly.
ChromaTwist Dye to CD8 Antibody Conjugation
A ChromaTwist dye has been conjugated to CD8 RPA-T8 antibody (Ab). Characterisation of the Gen-2-Dye●CD8 conjugate (post Pierce™ Dye Removal Column and Amicon Ultra-0.5 (30 kDa) centrifugal filtration) to rigorously exclude free dye) with absorbance (Figure 1a) and emission (Figure 1b) spectroscopies together with a Conjugate Check Kit (Figure 1c) provided evidence of successful conjugation, and removal of free dye. Degree of labelling of upto 10 CT dyes per Ab was achieved.
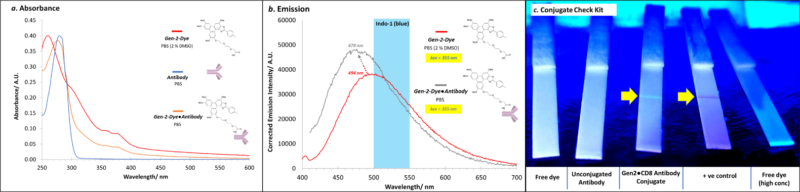
Furthermore, this blue emitting CT-Dye dye has been conjugated to the CD4 and CD8 antibody and the two conjugates were incubated with compensation beads. The headline result is that CT-Dye-Ab(CD8) and CT-Dye-Ab(CD4) produced spectral output with positive bead staining at both 355 nm (Figure 2a) and 405 nm (Figure 2d) excitation. An 18 hr incubation (37 °C) with the CT-Dye-Ab(CD8) conjugate achieved 40 % positive staining (Figure 2b–c), whilst a 30 min incubation (4 °C) with the CT-Dye-Ab(CD4) conjugate achieved 23 % positive staining (summarised in Table in Figure 2e–f)). Full spectral histograms with both 355 nm and 405 nm excitation display a bimodal bead distribution most prominantly in the UV-7 [514/28 nm] (Figure 2b–c) and V5 [508/20 nm] (Figure 2e–f) channels, respectively.
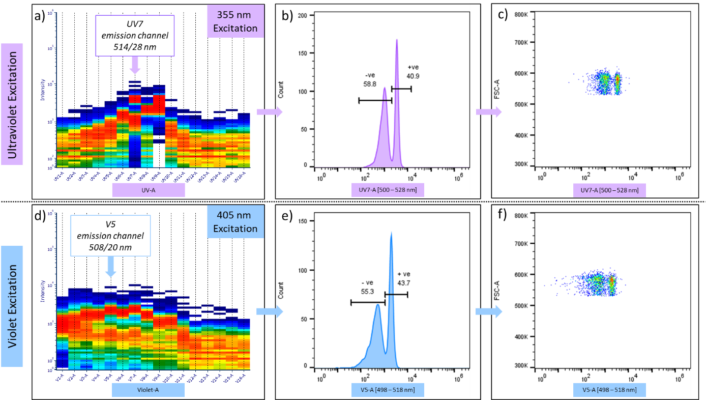
| Conjugate | Data | Ultraviolet Excitation (355 nm) | Violet Excitation (405 nm) | ||
| – negative (%) | + positive (%) | – negative (%) | + positive (%) | ||
| CT-Dye-Ab(CD8) (37 °C, 18 hr) | Shown Above | 58.8 | 40.9 | 55.3 | 43.7 |
| CT-Dye-Ab(CD4) (4 °C, 30 min) | Not Shown | 76.2 | 23.5 | 73.4 | 25.2 |
